Information about KVO3, NaVO3 & NH4VO3 compounds
KVO3 — Potassium metavanadate is an inorganic compound, potassium and vanadium acid salt, in form of colorless crystals, dissolves in water, forms crystalline hydrate. Has more density than water. Contact with the body might be dangerous.
NaVO3 — Sodium metavanadate. Is also an inorganic compound, in form of a yellow crystalline substance, soluble in water, very hygroscopic. Toxic, its maximum permissible concentration is 0.5 mg / m³
NH4VO3 — Ammonium metavanadate. Not a natural compound. Ammonia and metavanadic acid salt, usually has a white color, although a slight yellow is not uncommon.
Ammonium Metavanadate (AMV)
CAS No.: 7803-55-6
UN No.: 2859
Transport hazard class: 6.1
Application
- Colouring and glaze for ceramic industry.
- Pigment and absorption of ultraviolet light or infrared light for glass industry.
- DeNOx FCC additives for Petroleum Processing.
- Additive for li-ion battery material.
Specification
| NH4VO3 | 99.5% min |
| Si | 0.06% max |
| Fe | 0.025% max |
| S | 0.02% max |
| Al | 0.03% max |
| Cl | 0.008% max |
| As | 0.01% max |
| Na2O+K2O | 0.08% max |

Sodium Metavanadate (SMV)
CAS No.: 13718-26-8
UN No.: 3285
Transport hazard class: 6.1
Used in
- Removal hydrogen sulfide for stretford & sulfolin gas processing (gas sweetening).
- Colouring and glaze for ceramic industry.
- As a pigment material of bright yellow for lacquer and plastic.
- Absorption of ultraviolet light for glass industry.
- Dye stuff for textile industry.
Specification
| NaVO3﹒2H2O | 99% min |
| K2O | 0.1% max |
| Fe | 0.05% max |
| S | 0.03% max |
| As | 0.01% max |
| P | 0.01% max |

Potassium Metavanadate (PMV)
CAS No.: 13769-43-2
UN No.: 2864
Transport hazard class: 6.1
Used in
- A vanadium source for CO2 gas removal processes using a potassium carbonate-based vanadium-rich scrubber solution, such as the Benfield process or Catacarb process.
- Catalysts for sulphuric acid.
- Absorption of ultraviolet light for glass industry.
Specification
| KVO3 | 99% min |
| Na | 0.3% max |
| Fe | 0.04% max |
| S | 0.05% max |
| As | 0.03% max |
| P | 0.02% max |

Packing


Size
Highness: 0.45 m;
Diameter: 0.35 m;
Weight: 2.3 kg ~ 2.6 kg
Potassium metavanadate (KVO3)
Physical properties
Potassium metavanadate is a colorless to pale green solid. It is a crystalline structure that is denser than water, when it comes in contact with the skin it causes irritation. It is advised to wash in running water in case it gets into the eyes or the skin, as the first precaution measure. Potassium metavanadate is toxic when ingested hence one should be careful when coming in contact with it. This compound is known to be a non-combustible substance however it decomposes upon heating to produce fume that is corrosive and toxic. This chemical compound is known to be soluble in water. It is a salt that is basic in PH.
Chemical properties
Potassium metavanadate is written as potassium;oxido(dioxo)vanadium in IUPAC it has a molecular weight of 138.07 g/mol it has been found to be a hydrogen acceptor with three counts. It is a compound that has two covalent bonds. Potassium metavanadate has the molecular formula KO3V. The compound has a melting point of 520 oC.
2D structure of potassium metavanadate
The compound is a weak reducing agent hence it reacts with both weak and strong reducing agents. If heated in a container that is sealed it can explode and pollute the environment.
Protective measures
Medical personnel working in a place where it is manufactured or used should be aware so that they can be ready in case of an emergency. However, the workers should also be taught the first-aid measure that they can take in case of an emergency. Some first aid measures may include: Moving the person to an open-air or outside to a place with open-air, to give the person artificial respiration, and not using the mouth-to-mouth method since that may cause one to be a victim when the compound is inhaled or ingested, lastly to administer oxygen when the person has a breathing problem. Clothes or shoes that may be contaminated should be removed and kept away. In case of fire containers containing the substance should be moved away (ChemicalBook). Fires produced from this compound can be put off using any class of fire- extinguisher. Personnel should not touch a container that is spilling out the compound unless they are wearing protective clothing. In case of a leak, the spilling Potassium metavanadate should be prevented from getting into sewer lines, basement, or any confined area. It should be covered with plastic or dry earth sand in case of spillage. When preparing the compound one should wear protective clothing as recommended.
Uses
Potassium metavanadate is used as a corrosion inhibitor and anti-scaling agent. It is also used in companies that make dyes, ink and compounds that make clothes colors
Sodium Metavanadate (NaVO3)
Physical properties
Sodium metavanadate is an inorganic, yellow compound with a water-soluble crystalline structure. It has a higher density than that of water and it is soluble in water. It is a basic compound that will react with an acidic solution in an aqueous mixture. The compound emits acrid smoke and fumes when it is heated to decomposition. It is toxic if ingested and has a category 3 oral toxicity. Acute toxicity will arise if the compound is inhaled and it causes irritation when it comes in contact with the skin or the eyes. It has chronic, toxic effects on aquatic life.
Chemical properties
The IUPAC name for sodium metavanadate is sodium;oxido(dioxo)vanadium. Its molecular weight is 121.928 g/mol and no formal charge is present. It has a melting point of 630 °C and it has a hydrogen bond acceptor count of three. It is a compound that has two covalent bonds and complexity of 49.8. Sodium metavanadate has the molecular formula NaO3V.
2D structure of potassium metavanadate
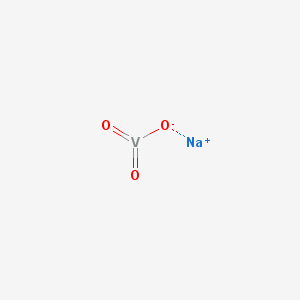
Sodium methavanadate is a moderately strong oxidizing agent.
Protective measures
Personnel who work at an area where the neat of the chemical is weighed and diluted, should wear a certified face respirator that has been outfitted with a specialised gas cartridge and a dust filter. Sodium methavanadate should be store in a refrigerator in a secured location. In the case of a small spill or leak in its solid form, small amounts of water should be added to spill material, afterwhich the dampened material is discarded into the proper container. To pick up any residual spill, a moist absorbent paper can be used. Dispose of the absorbent paper in a sealed plastic bag. Any clothing or clotheswear that has been contaminated should be properly disposed of. All surfaces affected by the spillage must be washed down with a soap and water solution. The contaminated area should remain off limits or quarantined until the relevant superior personnel has verified that the area has been efficiently sterilised. For larger spills, a circular area around the spill or leak should be established, with a diameter of a 50 meters (150 feet) for liquids and least 25 meters (75 feet) for solids as precautionary measure. If the vehicle carrying the compound is affected by a fire, the area around it should be isolated for about 800 meters (1/2 mile) in all directions. If the eyes are affected, the victim’s eyes should be checked for the presence of contact lenses and be removed if it is present. A saline solution can be used to rinse the eye for 20 to 30 minutes. A hospital or poison control center must be contacted as soon as a victim of contamination has been identified. No medical treatment of the victim must occur without specific instructions from a medical personnel. Vomiting should not be induced if sodium metavanadate is ingested, and the affected person should be immediately transported to a hospital.
Uses
It can be used to treat certain conditions of the body such as diabetes and high cholesterol.
Ammonium Metavanadate (NH4VO3)
Physical properties
Although typically a white solid, it is often yellow owing to the presence of impurities of V2O5. It is lightly soluble in water and has a density of 2.326 g/cm3, making it denser than water. It is moderately toxic and may release toxic fumes. If the compound is heated, it loses ammonia. It has long been suspected of causing genetic defects as well as cancer in humans. It is toxic or even fatal when it is inhaled. It causes serious irritation when it comes in contact with the skin or the eyes.
Chemical properties
Ammonium metavanadate is an inorganic compound with the molecular formula NH4VO3 and a molecular weight of 116.977 g/mol. The IUPAC name for it is azanium;oxido(dioxo)vanadium. It has a hydrogen bond acceptor count of three as well as a hydrogen bond donor count of one. It has a complexity of 36.5 and has two covalent bonds. It is stable under recommended storage conditions. It has a pH below 7.0 in an aqueous solution, meaning it will react to neutralize a base. These neutralizations generate heat, but the generated heat is less than that which is generated by the neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. Ammonium metavanadate is a weak oxidizing agent. It is capable of reacting with a reducing agent to generate heat or by-products that are flammable, combustible, or otherwise reactive.
2D structure of ammonium metavanadate
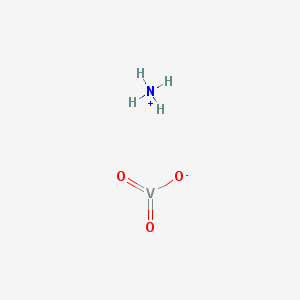
It takes the form of a polymeric structure consisting of chains of [VO3]-, formed as a VO4 tetrahedra. These chains are linked by hydrogen bonds with ammonium ions. It decomposes at temperatures above 70 °C.
Protective measures
It is a non-combustible substance that does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. When the compound comes in contact with metals, flammable hydrogen gas may be given off as a by-product and containers carrying the compound may explode when heated. Water runoff from fire control or dilution water of the neat of the compound may be toxic and cause environmental pollution. Containers containing ammonium vanadate should be kept tightly closed in an area free of moisture, since the compound is moisture sensitive, and it should also be well-ventilated. Should someone fall a victim of exposure to ammonium metavanadate, the medical personnel that are on the scene should be made aware of the material(s) involved and take the necessary precautions to keep themselves safe. The victim should be moved to fresh air if possible. Artificial respiration of the victim can be done if the victim is not breathing. Mouth-to-mouth method of resusication is not to be done as the rescuer can end up being another victim of the ammonium metavanadate poisoning. Artificial respiration should be done with the help of a mask equipped with a one-way valve or other proper equipment. Oxygen can be administered to the victim if breathing is still difficult. Contaminated clothing and gear should be removed and put in an isolated area. If the skin or eyes come in contact with this toxic substance, flush skin or eyes with running water immediately for 30 minutes. Proper regulation procedure and rules in regards to storage, transportation, treatment and disposal of the waste must be adhered to for safety reasons.
Uses
Vanadium is often purified from aqueous extracts of slags and ore by selective precipitation of ammonium metavanadate. The material is then heated to give vanadium pentoxide:
2 NH4VO3 → V2O5 + 2 NH3 + H2O
Ammonium metavanadate is also used in the preparation of Mandelin reagent. Madelin reagent is a qualitative test for the presence of alkaloids. It is also used as a dryer for paints and inks, and for dyes.
